In 1908, Sir Archibald Garrod, a British physician, proposed that several lifelong inherited conditions, including alkaptonuria, pentosuria, albinism, and cystinuria, were caused by defects in specific biochemical pathways, resulting in diminished or absent activity of certain enzymes. He termed these conditions "inborn errors of metabolism." Although his classification of cystinuria was later found to be inaccurate, Garrod's work provided the field of biochemical genetics with a strong foundation, leading to the rapid expansion of the list of inherited metabolic disorders.
Today, metabolic disorders are no longer restricted to rare genetic diseases. Common metabolic disorders, such as nonalcoholic steatohepatitis, kidney diseases, obesity, cardiovascular diseases, type 2 diabetes/prediabetes, and type 1 diabetes, represent a significant public health burden. Endocrine and metabolic diseases encompass a broad spectrum of conditions, including bone diseases such as osteoporosis, cystic fibrosis, thyroid disease, overweight, gynecological-endocrine, and lipid disorders, which affect millions of individuals and can severely reduce their quality of life. The impact of these common metabolic disorders is particularly severe in developing countries, where they are chronic, costly to treat, and frequently affect economically active populations.
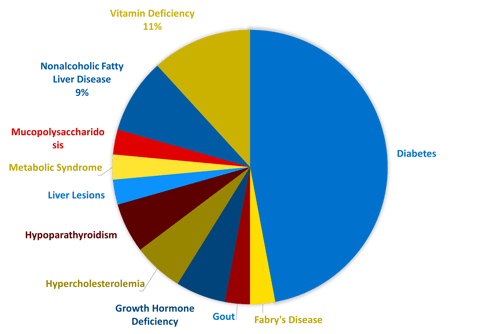
Endocrine and metabolic disorders are complex and often involve the regulation of multiple bodily functions. In addition to research and development, a thorough understanding of the underlying biology is essential for investigators and medical monitors conducting clinical trials. These professionals require expertise in a diverse range of conditions, as well as an understanding of the psychological aspects of working with this patient population. One of Linical’s core focus areas as a CRO is developing new drugs for metabolic and endocrine disorders. In recent years, Linical has been involved in nearly 30 trials, primarily large, international studies, testing innovative drugs for various metabolic disorders, including rare diseases. The accompanying pie chart illustrates the range of metabolic pathologies that we have worked with.
Linical's Recent Endocrinology and Metabolic Disease Experience
Challenges in Enrolling Endocrine/Metabolic Patients for Clinical Trials
Not surprisingly, the enrollment process is a critical challenge in clinical studies, regardless of the therapeutic area. However, in the field of endocrine and metabolic disorders, recruiting appropriate subjects can be particularly challenging. In fact, slow recruitment is a primary cause of delay in over 80% of global trials, with more than half being delayed by 1-6 months. This delay results in a loss of 85-95% of days in a clinical trial and is a leading cause of missed clinical trial deadlines.
Numerous factors contribute to the slow recruitment of patients with endocrine and metabolic disorders. One of the primary reasons is that many of these conditions are chronic and require ongoing specialist monitoring, which can make it challenging for healthcare providers to engage in running a clinical trial and recruiting patients.
Furthermore, for practitioners who become engaged in running a clinical trial and recruiting patients, their financial reimbursement per patient can, in some cases, be less than they would receive from regular practice. In addition, there is a financial disincentive for physicians to refer their patients to clinical trials. Physicians who do so must often refer those patients away from their care; thus, each patient referred represents a lost revenue stream and reduces physician referrals of patients to clinical research studies.
In addition, clinical trials often require patients to temporarily leave the care of their regular doctor and receive services from unfamiliar providers. This interruption of care can be undesirable, especially if patients are receiving a high level of medical care in high-income countries. Consequently, it is understandably difficult for many patients to justify the physical and emotional strain of leaving their regular provider to participate in a clinical trial.
Studies show that several barriers are perceived as relatively problematic by patients. These include missing work, the length and frequency of appointments/procedures, the number of appointments/procedures, access to the study location, and physical discomfort associated with the procedures. Patients are also often unaware of the possibility of enrolling in a clinical trial, and even when they are aware, it can be challenging to locate a trial. In addition, there is a general mistrust of industry-sponsored trials among the public, which further complicates the decision about whether to join a trial. Furthermore, the extensive paperwork associated with the informed consent process can be confusing and burdensome, creating additional barriers for patients.
Another contributing factor to the slow recruitment of patients with endocrine and metabolic disorders is the prevalence of rare diseases within these populations, which can be difficult to diagnose and often require the inclusion of pediatric patients in clinical trials. Additionally, certain bone metabolic diseases, such as osteoporosis, are more prevalent among geriatric populations, necessitating close monitoring by experienced investigators and medical monitors with high levels of expertise in metabolic disorders.
Solutions and Keys to Success for Endocrinology and Metabolic Clinical Trials
To address the challenges associated with enrolling patients into endocrinology studies, our primary focus is on finding a solution that directly resolves or improves upon these issues. By embracing the globalization of clinical research, we can effectively overcome these challenges and enhance the process of enrolling patients in endocrinology studies. Expanding trials to emerging regions such as the Asia Pacific, South America, and Eastern Europe, in addition to high-income countries, offers immense potential for mitigating these obstacles. This approach brings several advantages, including lower overall development costs, accelerated timelines, and access to larger patient populations. Furthermore, global clinical trials offer the opportunity to expand the pool of study participants in regions where current therapies may not be readily available. By conducting research in various regions, we can not only improve patient enrollment but also provide access to patient populations who may otherwise be excluded. This expansion of participant diversity strengthens the validity and generalizability of the study results. Moreover, global clinical trials enable the simultaneous launch of new medical products in multiple regions worldwide. By conducting research in different areas, sponsors can lay the necessary groundwork for regulatory approvals in those specific regions, streamlining the process and accelerating the availability of new treatments. Therefore, it is crucial to select a Contract Research Organization (CRO) with a strong presence in these regions and extensive experience in the therapeutic area to ensure successful trial conduct and maximize the benefits of global clinical trials.
Patient education, particularly through community-based physicians, social media, and collaborations with patient advocacy groups, presents a significant opportunity to address the recruitment challenges faced by chronic comorbid patients. By providing educational resources and raising awareness about clinical trials, trust can be built and fears alleviated among patients who may have concerns about leaving their current care provider or who are unaware of available trials. Patient advocacy groups play a crucial role in these efforts, serving as trusted sources of information and support for patients. Through collaboration, educational materials can be developed and disseminated, awareness campaigns can be organized, and the networks of patient advocacy groups can be utilized to reach a broader audience. This collaborative approach helps bridge the gap between patients and the medical community, addressing concerns, providing support, and increasing patients' knowledge about research opportunities. By involving patient advocacy groups, community-based physicians, and leveraging social media platforms, patients with chronic comorbid conditions can be effectively educated and engaged, ultimately improving recruitment rates and ensuring their awareness and willingness to participate in important clinical research. As previously mentioned, private practice physicians face disincentives when referring their patients to clinical trials. This lack of physician involvement in the development and implementation of clinical trials can compromise the scientific integrity of medical practice. Providing adequate compensation to physicians for patient referrals to clinical trials may increase recruitment rates in high-income countries, such as the United States. Encouraging community-based physicians to actively participate in clinical trials can also enhance community engagement in crucial research, increase the likelihood of physician adherence to evidence-based medicine, and improve patient recruitment rates.
In the realm of common metabolic diseases, having knowledgeable staff throughout the entirety of a clinical trial is of utmost importance. A well-trained and intelligent data monitoring committee is particularly vital, as they are responsible for developing appropriate analytic methodologies, considering ethical and operational challenges associated with non-inferiority designs, and robustly evaluating the contribution of adverse effects, such as hypoglycemia or weight gain, to observed clinical outcomes. Additionally, the committee evaluates interim trial results, which is a commonly used practice in this therapeutic area.
In summary, the recruitment of suitable subjects for clinical trials represents a significant challenge, especially in the field of endocrine and metabolic disorders. Collaborative efforts between healthcare providers and clinical researchers are crucial to overcome the various barriers that impede recruitment, including financial disincentives, patient burden, and lack of awareness and trust. By addressing these obstacles, clinical trials in the field can enroll appropriate subjects more efficiently and expedite the development of much-needed therapies for these conditions.
Tackling these challenges is a pivotal aspect of enhancing the caliber of clinical trials and ultimately improving the outcomes for the countless individuals suffering from metabolic disorders, both presently and in the future. At Linical, we are honored to be an integral part of this demanding yet exhilarating therapeutic area and remain committed to playing our part in aiding millions of patients globally.
Author:
Diana Davydov, MD
Associate Medical Director & Endocrinologist - Linical
References:
- Metabolic disease - britannica.com
- Certified Clinical Research Professionals Society - ccrps.org
- Social Science & Medicine 68 (2009) 1069–1074
- Challenges and opportunities in global clinical trials - pharmaphorum.com
- Transforming clinical research in the United States. challenges and opportunities - nap.edu
